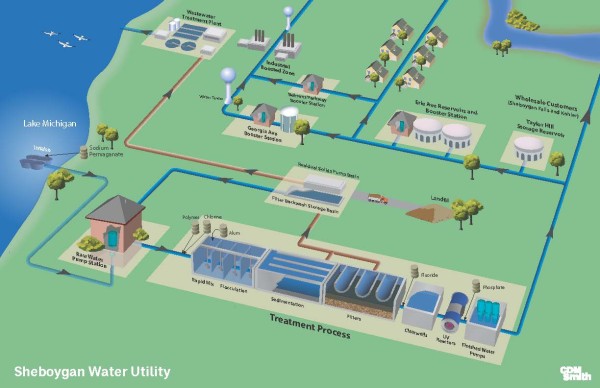
UV Disinfection
On August 1, 2016, the Sheboygan water Utility began operation of its Ultraviolet Disinfection Facility (UV). UV disinfection is an effective disinfection barrier for the inactivation of specific harmful microorganisms: E.coli, Cryptosporidium and Giardia. This technology also improves the multiple barrier approach with conventional treatment operations to eliminate a number of other naturally occurring pathogens in your drinking water (viruses, bacteria and protozoa).
Ultraviolet means “beyond violet” (from Latin ultra, “beyond”), violet is the color of the shortest wavelength of visible light. Ultraviolet light is a form of light that is invisible to the human eye. Microorganisms in the water are exposed to ultraviolet light when they pass by the submerged lamps. The UV light energy instantly destroys the genetic material (DNA) within bacteria, viruses and protozoa, thus eliminating their ability to reproduce and cause infection. Unable to multiply, the microorganisms die and no longer pose a health risk.
Coagulation
Coagulation is a process for increasing the tendency of small particles in an aqueous suspension to attach to one another and to attached to surfaces such as the grains in a filter bed. It is also used to remove certain soluble materials by absorption or precipitation. The coagulation process typically includes promoting the interaction of particles to form larger aggregates. Coagulation is important for removing microbiological contaminants and natural organic material from raw water.
Coagulation is a complex physical/chemical process whereby a stable suspension of particles is transformed into an unstable suspension. Particles that might have existed in lake water for months or years as stable, discrete units can be aggregated in an hour or less following successful destabilization.
Most particles in lake water have negative electrically charged surfaces. Because like charges repel, the forces of electrical repulsion can create stable suspensions of particles. Surface charge neutralization is one of the primary destabilization mechanisms involved in coagulation. By adding a chemical (such as aluminum sulfate) that reduces or neutralizes the negative surface charges, we can reduce or eliminate the electrical repulsion forces. Upon the creation of an unstable suspension, the unstable particles are then encouraged to aggregate into larger clumps of unstable particles.
Flocculation
Flocculation is a process to promote the interaction of unstable particles and form aggregates that can be removed through sedimentation and filtration. The goal of flocculation is to enhance the mechanisms that cause relative motion and collision between particles in a destabilized suspension. In practice, WTP's achieve flocculation by using mechanical mixers along with flow baffles to enhance gentle mixing. The term "floc" refers to the cloudy particles often described as snowflakes that begin to form as the unstable particles aggregate into a more visible form.
Sedimentation
Sedimentation is a solid-liquid separation process that occurs after coagulation and flocculation. It relies on the force of gravity. In general, floc will settle if it has time to travel the vertical distance from its location to the bottom of a sedimentation basin before it flows out of the basin. The terminal settling velocity depends on a number of factors. Sedimentation basins are designed to allow enough retention time for settling to occur.
Filtration
Filtration is the process of removing the remaining residual solids in water after sedimentation. In conventional WTP's, rapid sand (granular bed) filters are commonly used for filtration. The layers of a filter are designed to achieve an optimization of factors including solids removal effectiveness, filter run time, and energy loss. The upper layer of a filter typically contains larger media with larger pore spaces the lower layers of a filter contain smaller media with smaller pore spaces. This gradation of pore spaces allows incoming solids to be captured throughout the filter column instead of simply clogging the upper layers. When the pore spaces do become clogged, the filter must be backwashed to remove the captured solids.
Sodium Hypochlorite Chlorine
Sodium Hypochlorite Chlorine For most of the twentieth century, the Sheboygan Water Utility used elemental chlorine gas as its primary disinfectant. In later years, the Utility typically stored 8,000 to 10,000 pounds of chlorine gas on the premises. Despite its effectiveness as a disinfectant, chlorine gas is extremely hazardous in its concentrated form. Due to safety concerns, the Utility switched to liquid sodium hypochlorite (NaOCI) as its primary disinfectant in 2001.
Elemental chlorine gas and sodium hypochlorite both form hypochlorous acid (HOC1) and hypochlorite ion (OCI1) as its primary disinfectant in 2001.
Elemental chlorine gas and sodium hypochlorite both from hypochlorous acid (HOC1) and hypochlorite ion (OC1-1) when added to water. Taken together, the concentration of HOC1 and OC1-3 is defined as the free available chlorine. Free chlorine is a very strong disinfectant used to kill a number of micro-organisms.
The Utility typically injects sodium hypochlorite early in the treatment stream. This allows ample time for the disinfection process. Also, if the incoming raw water exhibits a large chlorine demand, sodium hypochlorite can be injected at later stages in the treatment process.
Sodium hypochlorite and other sources of chlorine can react with natural organic matter in water to produce tri-halomethanes and other organic compounds of concern. Municipal water is heavily regulated to ensure compliance with established safe levels of so-called disinfection by products (DBPs). Water produced at the Sheboygan Water Utility far exceeds all of these requirements.
Municipal water utilities must maintain a free chlorine residual within the distribution system. This helps to ensure disinfection throughout the water system and right up to the tap. Water leaving our plant typically has a free chlorine residual ranging from 0.5-1.0 parts per million (ppm).
Ortho-Phosphate
Before 1942, most private water laterals were constructed of lead or galvanized pipe. In many Wisconsin cities (including Sheboygan), some of these older service laterals are still active. Most new private water laterals are constructed of copper pipes.
Water tends to sit in water laterals when water consumption is low. Therefore, the water has time to corrode (or dissolve) the internal surface of the lateral pipes. In this process, small quantities of dissolved material from the pipe can be carried into the home. Although rarely the sole cause of lead poisoning , dissolved lead from lead water pipes can increase a person's total lead exposure, particularly for infants who drink baby formulas mixed using the water.
In the early 1990's, the Sheboygan Water Utility (and others in the state) began a testing program to determine the extent of lead and copper corrosion of private water laterals. WDNR and EPA began public educational programs to inform homeowners about health issues concerned with corrosion of lead in older water laterals. Lead-based solders were also banned for potable water systems.
In 1995 the Sheboygan Water Utility began adding small quantities of orthophosphate (0.33 parts per million) to its treatment process. Orthophosphate forms insoluble passivating films on the inside of metal pipes. These thin films reduce the ability of water to corrode the pipe material and thereby reduce the possibility of dissolved lead coming from older water services.
The Sheboygan Water Utility continues to monitor for lead and copper corrosion of water laterals. In addition, homeowners are now required to replace their old lead water services during street and water main reconstruction projects.
Potassium permanganate (KMnO4) has been used as a water treatment oxidant for decades. The Sheboygan Water Utility began using permanganate in 1981 to help control natural tastes and odors in the water. Permanganate also provides limited disinfection capability.
Tastes and odors occur from a variety of sources including algae, actinomycetes, and various sulfides. In Sheboygan, tastes and odors generally do not pose a problem until the raw water temperature exceeds 50 F - usually in the months of July and August. At such times and under certain conditions, the raw water can infrequently acquire a slightly musty taste that is harmless but undesirable.
Recently, the Utility has also used permanganate to help deter zebra mussels from colonizing in the water intake pipes. Permanganate is generally fed at very low rates of 0.15 parts per million.
Fluoridation
In 1946, the Sheboygan Water Utility became the first in Wisconsin to fluoridate municipal drinking water (and third in the United States) for the prevention of tooth decay.
For many years, the Utility fed a powdered form of fluoride. In 1996, the Utility switched to liquid hydrofluosilicic acid. This is fed at 0.70 parts per million (ppm), which is the minimum within the recommended range for drinking water. In recent years, due to ongoing research, the recommended dosage rate has been reduced. By comparison, most toothpastes have a fluoride concentration of more than 1,000 ppm, but these are not intended for ingestion.
The American Water Works Association (AWWA) supports the fluoridation of municipal water as a public health benefit, while also committing itself to regular review of current research on fluoridation.
The Wisconsin Dental Association and its more than 3,000 member dentist and dental hygienists are committed to promoting quality oral health care and support community water fluoridation. Additional information is available here: https://www.wda.org/.
Many of the major health institutions endorse the fluoridation of municipal water click here to read more.
Processes/Chemicals Used
Some of the different chemicals we use are:
• Aluminum Sulfate (Coagulation)
• Sodium Hypochlorite (Disinfection)
• Potassium Permanganate (Oxidation and zebra mussel control)
• Orthophosphate (Corrosion control)
Some Processes used are:
• UV Disinfection
• Coagulation
• Flocculation
• Sedimentation
• Filtration